Earth-friendlier ammonia
1
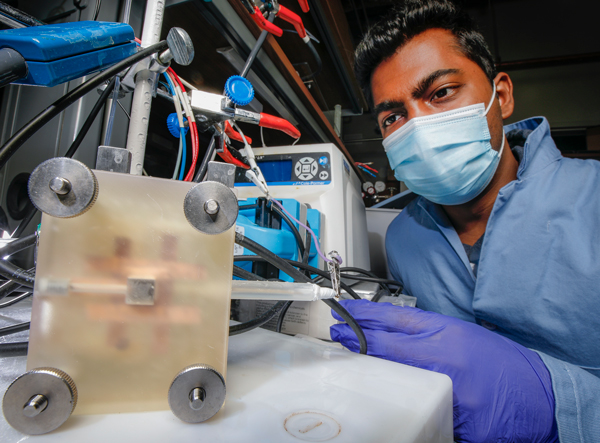
Ammonia is the second most commonly produced chemical in the world and an important component of most fertilizers, but in certain ways, it’s not great for the environment. The industrial processes needed to produce it generate several millions of tons of carbon dioxide—a potent greenhouse gas—each year.
Now, researchers led by Meenesh Singh, assistant professor of chemical engineering, have found a way produce ammonia with a potentially much lower carbon footprint.
Their solution lies in how to break apart nitrogen gas, one of the ingredients used to make ammonia. Its molecular bonds are very stable, meaning it takes a lot of energy to break them so that the nitrogen can bind to hydrogen and produce ammonia. Most manufacturers do that by burning fossil fuels to generate enormous amounts of heat, but that also creates byproduct greenhouse gases.
Singh and colleagues have developed a new method using a mesh screen coated in copper, a catalyst that helps to bind nitrogen. The electrification of the screen helps to drive the reactions. While energy is still needed, Singh said the new approach requires far less fossil fuel than traditional methods: just enough to electrify the screen. Right now, the process yields 20 percent ammonia and 80 percent hydrogen gas, but the research team sees great future promise in its efforts.