UIC chemical engineers get ‘muddy’ to develop renewable fuel cell
Text block one Heading link
Engineers digging for efficient ways to harness sustainable power found a surprising fuel source — mud and a common bacterium often found in it.
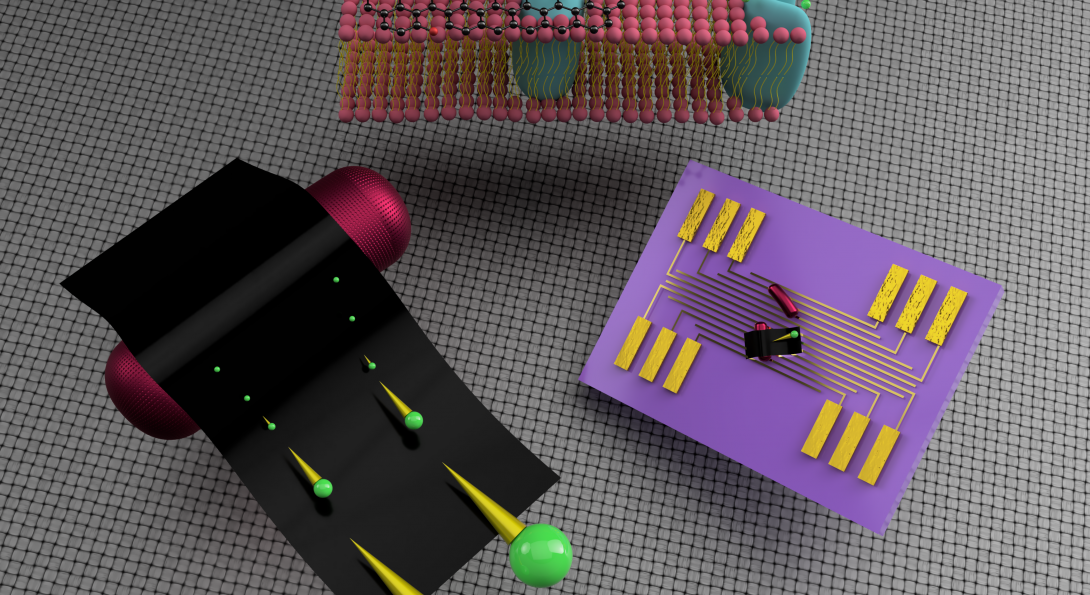
University of Illinois Chicago researcher Vikas Berry and his colleagues built a fuel cell from electrogenic microbes covered in extremely thin graphene sheets. The bacteria produce electrons on their surface and release protons as they consume nutrients from the mud, such as sugar or acetates. The fuel cell then separates the electrons in the anode and moves the protons to the cathode to produce green and renewable power.
Their work is detailed in the article “Interface of Electrogenic Bacteria and Reduced Graphene Oxide: Energetics and Electron Transport,” which is the cover story in the forthcoming edition of the journal ACS Applied Electronic Materials.
The mud’s bacterium has been used as electron-source since the 1980s when it was first discovered.
“For every molecule of acetate consumed, eight electrons are produced by this bacterium,” said Berry, UIC professor and head of chemical engineering. “However, previous work has fallen short of electrically connecting the cell surface with high-conductivity material.”
To get around this issue, the UIC engineers blanketed the bacteria with flexible and highly conductive graphene sheets to cover the cell surface to improve the efficiency of electron extraction. The graphene, therefore, directly extracts the electrons exiting from the electrogenic protein channels on the cell surface.
“That’s the ingenuity of this work. The graphene is single atom thick, so it is very flexible,” Berry said. “It covers the bacteria to capture more surface of the bacteria, which has the protein channels, where the electrons come out.”
Another positive attribute of this design is the renewable nature of the bacteria.
During the course of their research, Berry’s team worked with several other cell types that would die in 8 to 14 hours.
However, these cells are resilient and can survive harsh conditions in nature, so if food is continually added they will keep producing electrons until the food source runs out.
Research team member Sheldon Cotts, a UIC Ph.D. student in chemical engineering, said the bacteria is unique because it almost completely breaks down its source of food, so it could be used to break down organic wastewater streams from municipal wastes, breweries and some kinds of industrial waste.
“When they break these substances down, they may provide electricity to the grid. This is a rare example of a waste remediator and power source at the same time,” Cotts said.
The UIC researchers also discovered that when their graphene interfaced with these bacteria, graphene’s own conductivity improved, which helped the extraction of the electrons even further.
The group’s current work focuses on single-cell experiments to better understand the mechanism behind the electron-transport phenomenon and building microfluidic devices to harness the potential of the bacteria.
In the future, the fuel cells could be used to power microdevices, according to Berry.
“Since graphene has already revolutionized device circuitry, its ability to conformally blanket electrogenic microbes to harvest electrons can lead to new-generation designs of bio-powered circuitry to run stand-alone nanodevices,” Berry said. “As these cells feed on soil, such devices can be powered from the ground mud to power or charge medical, defense or other devices in remote areas.”
Cotts added that forthcoming work also needs to be done to educate the public on the fact that not all bacteria are dangerous and it can be used to address energy and other societal issues.
Co-authors on the article also include Bijentimala Keisham, UIC alumna who earned a Ph.D. chemical engineering, and Jay Rawal, UIC senior in chemical engineering.
Co-written by David Brazy