Research aims to understand cellular differentiation process for cell, tissue regeneration
block 1 Heading link
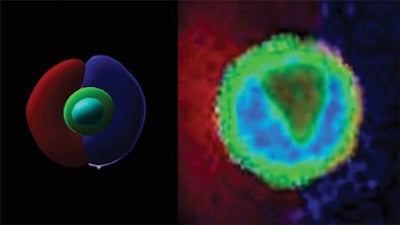
Stem cells generate all other cells with specialized functions. From these cells, come daughter cells that become new stem cells or specialized cells. However, how this fundamental biological process is regulated remains poorly understood.
“Stem cells are important cells that are used to regenerate tissues so there’s a lot of interest in terms of how to use them to restore damaged tissues,” said Jae-Won Shin, associate professor of pharmacology and regenerative medicine in the College of Medicine, Richard and Loan Hill Department of Biomedical Engineering associate professor, and corresponding author of a recently published paper on this topic.
Shin and his team engineered a new approach to encapsulate single stem cells in micro-engineered environments with asymmetrically distributed signals to control where the signals are placed around single cells in the 3D environment.
In previous approaches, it was very difficult to control where the signals are around single cells because the entire cell is surrounded by ligands or adhesion sequences, but this technology allows investigators to control where they’re placed after encapsulation of single cells.
This approach was previously done with 2D micropatterning and is difficult to control in 3D micropatterning.
“We were studying the 3D environment because it is important to understand cellular behavior and recapitulate what’s happening in a more physiological environment,” Shin said. This research can also help study biological questions including how single cells break symmetry in a 3D environment and how they respond to cooperative or competing biological signals from the environment.
In turn, Shin explained that instead of making individual cells surround the adhesion sequences that exist around individual cells, once he and his colleagues put the adhesion peptide into half of the cell, they saw how cells broke their symmetry.
“Cells elongate as opposed to expanding uniformly and also it introduces a signaling pathway that leads to the process called polarization or cell polarity, which is an essential process for stem cells to undergo specialization,” said Ik Sung Cho, a postdoctoral fellow in Shin’s lab and first author on the study. “We demonstrated that by simply putting the ligand on one side only, you can actually polarize cells and then differentiate them.”
Cell polarization can serve as a platform technology for people who are interested in studying this cellular phenomenon, which is broadly applicable to understanding differentiation and other type of cellular functions.
Shin and his team used this kind of approach for different purposes to understand both the fundamental sciences of cell biology that are relevant to the tissue regeneration process, but also to use them to deliver stem cell-based therapeutics into the body.
“The initial technological goal of the paper was to control where the cell signals that can control cellular behavior are placed in the grid environment or on single cells,” Shin said. “The biological goal is to try to understand how this teaching environment impacts the cellular differentiation process.”
Shin’s research will also help understand cancerous tumor formation as it relates to stem cells undergoing proliferation as specialized or unspecialized cells, as well as understand the development where cell-matrix interactions play an important role.
Shin and his colleagues published these findings in Advanced Science in a paper titled, “Deterministic Single Cell Encapsulation in Asymmetric Microenvironments to Direct Cell Polarity.”
This paper was also co-authored by Prerak Gupta, Nima Mostafazadeh, Sing-Wan Won, Saiumamaheswari Saichellappa, Stephen Lenzini, and Zhangli Peng, all of UIC.